We use the word “wet” every day to describe a towel, the ground, or our hair. But in physics and chemistry, “wetness” isn’t just a feeling it’s a measurable force. Here’s an answer-first guide explaining the science of wetting, the battle between adhesion and cohesion, and contact angles. We’ll break down why water makes paper wet but rolls right off a duck’s back.

The Science: Adhesion vs. Cohesion
To understand wetness, you have to look at the molecular battle happening in every droplet.
- Cohesion (Sticking together): Water molecules love to stick to each other because of hydrogen bonding. This force tries to pull the liquid into a spherical shape (a droplet) to minimize surface area.
- Adhesion (Sticking to others): This is the attraction between the liquid molecules and the solid surface they are touching.
The Verdict: “Wetting” happens when Adhesion > Cohesion. The water prefers the surface over itself, so it spreads out. This is central to the debate of is water wet technically, water is the wetting agent, but it needs a solid surface to interact with to create the phenomenon of wetness.
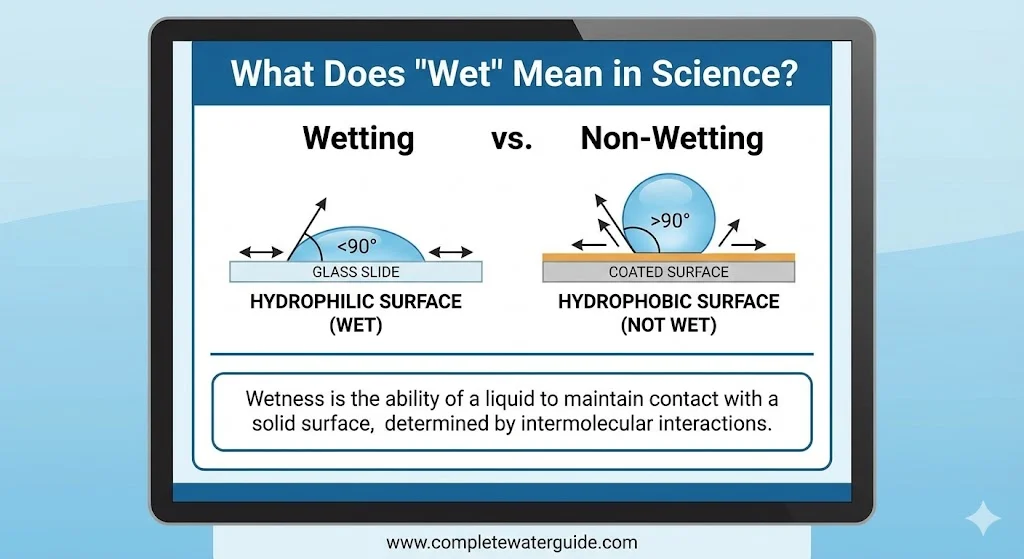
Measuring Wetness: The Contact Angle
| Contact Angle (θ) | Wettability State | Definition | Example |
|---|---|---|---|
| 0° | Perfect Wetting | Liquid spreads completely flat. | Water on clean glass. |
| < 90° | High Wettability (Hydrophilic) | Liquid spreads significantly. | Water on paper or cotton. |
| > 90° | Low Wettability (Hydrophobic) | Liquid beads up. | Water on Teflon (pan). |
| > 150° | Superhydrophobic | Liquid rolls off instantly. | Lotus leaf / Waterproof sprays. |
Scientists use a “goniometer” to measure this angle. Lower angle = Wetter surface.
Why Some Things Don’t Get Wet
Have you ever seen water roll off a duck’s feathers? That is hydrophobicity in action.
- Hydrophilic (Water-Loving): Materials like wood, cotton, and concrete have surfaces that chemically bond well with water. The adhesion is strong, so the water soaks in or spreads out.
- Hydrophobic (Water-Fearing): Materials like fats, oils, and waxes repel water. The cohesion of the water is stronger than its adhesion to the wax, so the water pulls into a ball to avoid touching the surface.
Are All Liquids “Wet”?
Not necessarily. “Wetness” depends on the pair of materials.
Example: Mercury.
If you pour liquid mercury onto glass, it does not wet the glass. It stays in a perfect ball. In scientific terms, mercury is a liquid, but it does not make glass “wet” because its cohesive force is massive compared to its adhesive force with glass.
FAQs
What is the scientific definition of wetting?
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions (adhesive forces) effectively overcoming the liquid’s internal cohesive forces.
Is ice wet?
Ice itself is a solid. It feels wet only when a thin layer of liquid water melts on its surface due to heat transfer from your hand. “Dry ice” (solid CO2) sublimates directly to gas, so it never feels wet.
Can you make water wetter?
Yes! By adding a surfactant (like soap or detergent), you reduce the surface tension (cohesion) of water. This allows the water to spread out more easily and penetrate fabrics better—that’s how laundry detergent works.
Is wetness a physical property?
It is a system property. It describes the interaction between a specific liquid and a specific solid, rather than being a property of the liquid alone.
References
- USGS — Adhesion and Cohesion of Water
- Chemistry LibreTexts — Cohesive and Adhesive Forces
- Encyclopaedia Britannica — Wetting
What Readers Say (Verified)
Verified
Excellent explanation of contact angles. I use this to explain surface tension to my high school students.
Verified
Finally understood adhesion vs cohesion. The mercury example was the lightbulb moment for me.
Verified
Very clear. I always thought ‘wet’ was just a sensation, didn’t know about the physics definition.
Verified
The table on wettability states is perfect. Concise and scientific.

2 Comments
Is Ice or Steam Wet? - completewaterguide.com
December 22, 2025[…] this, we have to look at the definition of “wetness.” As explained in our guide What Does Wet Mean in Science?, wetness is the interaction of a liquid adhering to a solid […]
Why water makes things wet - completewaterguide.com
December 22, 2025[…] we explored in What Does Wet Mean in Science?, wetting is a battle between two […]